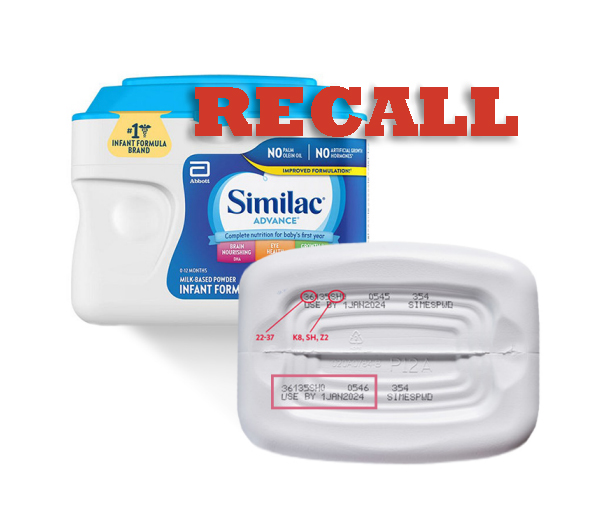
Abbott infant formula recall
FDA Warns Consumers Not to Use Certain Powdered Infant Formula Produced in Abbott Nutritions Facility in Sturgis Michigan News provided by. Abbott is recalling certain powdered infant formula products from the marketplace in Canada because of possible Cronobacter sakazakii and Salmonella contamination.
Food and Drug.
. Food and Drug Administration Feb 17 2022 1747. FDA Warns Consumers Not to Use Certain Powdered Infant Formula Produced in Abbott Nutritions Facility in Sturgis Michigan. February 17 2022 247 PM 4 min read.
Abbott Nutrition is voluntarily recalling specific lots of three infant formulas after four children were sickened and one died after being fed formula produced at the Sturgis Michigan plant. Abbott Laboratories has been directed to recall certain batches of one of its powdered infant formulas due to the possibility. The recall affects certain lots of Similac.
This recall was triggered by a. Abbott one of the countrys largest infant formula makers said it is recalling all potentially affected products manufactured at the facility. Several powdered infant formula products have been recalled by Abbott Inc following reports of four infants developing bacterial infections after consuming the products the US.

Similac Advance 20 Infant Formula 13 Oz Can Baby Formula Similac Complete Nutrition





/cloudfront-us-east-1.images.arcpublishing.com/gray/HL3DPQ3NZBFEBBTOBD4A3AUHVU.png)
